SEE Science (Classification of Elements) Notes
Classification of Elements
Matter, Elements & Compounds
Matter: - Anything which
occupies space and possesses mass is called matter.
For example: - Table, Chair, Human
being, etc.
Element: - An element is a
simplest substance which cannot be spited into simpler form.
An element is composed of same types of
atoms.
For example: - Gold, Zinc, Copper,
Silver, etc.
Compound: - A compound is a pure substance which is formed by the combination of two or more elements chemically in definite proportion by weight. A compound is composed of same types of elements.
For example: - water,
glucose, sugar, carbon dioxide, common salt etc.
Mixture
Mixture: - When two or more
substances are mixed together and they do not react chemically, then the
resulting mass is called mixture. Mixture is an impure substance.
For example: - Salt solution, Sugar
solution, Air, Soil, Muddy water etc.
Mixture may be homogeneous or heterogeneous.
Homogeneous Mixture: - The
mixture in which one of the components is not visible with naked eyes and
the components are mixed in such a way that every sample of mass is
uniform is called homogeneous mixture.
For example: - Salt solution, Sugar
solution etc.
Heterogeneous Mixture: - The
mixture in which one of the components are visible with naked eyes and every
sample of mass is not uniform is called heterogeneous mixture.
For example: - Soil, Muddy water, Smoke
in air, Seed coats in rice etc.
Atoms & Molecules
Atom: - The smallest particle of
an element which cannot be further sub-divided and takes part in a chemical
reaction is called an atom. Atoms of an element are identical in all respect
and may or may not exist freely in nature.
For example; H, O, N, C, B, F, I, K,
P, S, U, Fe, Cu, Hg etc.
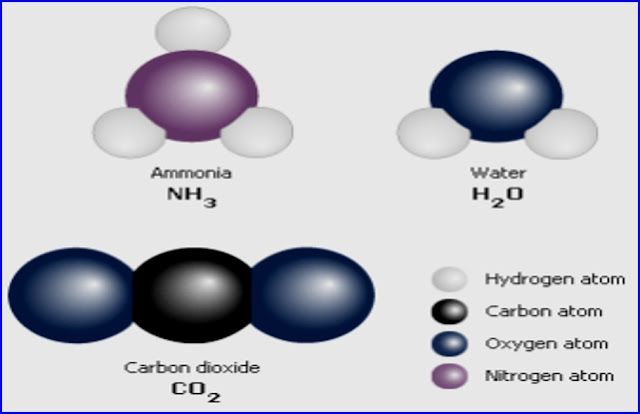
Molecule: - The smallest particle of
an element or a compound which can exist freely in nature is called a molecule.
Molecules of an element or compound are identical in all respect.
For example: -
•Molecules of elements: - H2,
O2, N2, I2, Cl2 Br2 etc.
•Molecules of compounds: - CO2,
H2O, NaCl, HF, KBr, CuSO4 etc.
Symbols
Symbol: - The short form representation
of an element is known as a symbol. A symbol indicates for one atom
of an element. The symbols are given by using one or two letters of the
name of elements.
Symbols are derived in different ways:
a. By using single letter of name
[Capital letter]:
b. By using double letters of the name
[First Capital and second small letter]:
c. Some symbols are derived from their
Latin names:
Radicals or ions
The atoms or group of atoms which carry charge on
them and behave as a single unit during chemical reaction are called
radicals. They are also called ions.
Radicals are of two types: -
1. Acid radicals or
Electro-negative radicals :- The atoms or group of atoms which
carry negative charge on them and behave as a single unit during
chemical reaction are called acid radicals or electro-negative radicals.
2. Basic radicals or
Electro-positive radicals :- The atoms or group of atoms which carry positive
charge on them and behave as a single unit during chemical reaction are
called basic radicals or electro-positive radicals.
Details of Elements
Atomic Structure of Elements
Sub Atomic Particles
Atoms are
smallest particles of an element. They are composed of further smaller
particles which are called sub-atomic particles. There are more than 40
kinds of sub-atomic particles. Among them electrons, protons and
neutrons are called fundamental sub-atomic particles.
Electrons: Electrons are negatively charged particles with negligible mass. An electron has mass equal to 1/1839th mass of one atom of hydrogen (or 1/1839th of 1 amu). Electrons are revolving around the nucleus in different orbits (shells).
An electron is represented by e-.
Protons: Protons
are positively charged particles with mass of 1 amu. A proton has mass
equal to the mass of one atom of hydrogen. Protons are situated in the
nucleus.
A proton is represented by p+.
Neutrons: Neutrons are neutral particles with no charge. A
neutron has mass equal to the mass of one atom of hydrogen (1 amu).
Neutrons are situated in the nucleus.
A neutron
is represented by n°.
Nucleons: The protons and neutrons are located
together in the nucleus. Therefore, collectively they are called nucleons.
Valency
Valency of an element may be defined as the
number of Hydrogen atoms, number of Chlorine atoms or double the
number of Oxygen atoms that can combine with one atom of that
element.
Variable Valency
Some of the elements show more than one value of
valency. This type of valency is called variable valency. The lower
valency of an element is indicated by writing –ous at the end of the
name of the compound. Similarly, The higher valency is indicated by writing
–ic at the end of the name of the compound. Some examples are,
Molecular Formula
H2, O2, N2, F2,
Cl2, Br2, I2, P4 etc. are the
molecular formulae of related elements.
H2O, CO2, HCl, CO, NO2,
NH3, CaCO3, HNO3, H2SO4,
NaCl, AgCl, K2O, H2S, MgO, AlCl3, MgCO3,
Na2SO4, HgO, CuSO4, ZnSO4 etc.
are the molecular formulae of some compounds.
So, molecular formula are the smallest particle
of an element or compound that can exist independently.
How can we write molecular formula?
We can write a molecular formula easily by using criss-cross method.
Step 1: The symbols or ions are written side by side.
Electro-positive radical on the
left and electro-negative radical on the right side. The valency is
written on right top as
superscript. If the radical is a compound radical then valency is written outside the bracket.
For
example: -
Calcium
Carbonate
Ca2
(CO3)2
Step 2: The valencies are exchanged to
opposite radicals by criss-cross method
Ca2 (CO3)2
Step 3: LCM of the valencies is taken if
necessary.
Ca1
(CO3)1
Step 4: The symbols of both radicals are combined.
Ca1(CO3)1
CaCO3
Hence the molecular formula of calcium carbonate
is CaCO3.
Molecular Structure
The location of
the atoms, groups or ions relative to one another in a molecule, as well as the
number, type and location of covalent bonds is called molecular structure.
Physical Change & Chemical Change
|
S.N. |
Physical
Change |
Chemical
Change |
|
1. |
It is a
temporary change. |
It is a
permanent change. |
|
2. |
Chemical
composition is not changed. No new substance is formed. |
Chemical
composition is changed. New substance is formed. |
|
3. |
Can be
reversed back easily. The component substances can be separated easily. |
Cannot
be reversed back easily. The component substances cannot be separated easily. |
|
4. |
It
takes place with low change in energy. |
It
takes place with high change in energy. |
|
5. |
Examples:
- Making sugar solution, Salt solution, Grinding rice, Breaking rocks,
Changing water into ice, Moving vehicles. |
Example:
- Burning of fuels, Cooking food, Digestion of food, Changing milk into curd,
Clotting of blood, Rusting of iron. |
Physical Methods For Separations of Mixtures
Some
solid substances like camphor, iodine, ammonium chloride etc. are converted
into gaseous state on heating and on cooling are changed back into solid state
without changing into liquid state. This phenomenon is called sublimation.
The
mixture of sand and camphor can be separated by this process.
The
mixture of common salt and iodine can be separated by this process.
When
the solvent from a saturated solution is partly evaporated and the resulting
solution is cooled, pure solid crystals are formed from the solution. This
phenomenon is called crystallization.
Pure
crystals of copper sulphate are obtained impure copper sulphate by this
process.
Common
salt is obtained from the ocean by this process.















hello
ReplyDelete